Food and Drug Administration FDA for approval of their mRNA vaccine to prevent COVID-19 in individuals 16 years of age and older. Granted by the FDA on December 11 2020.
Https Www Fda Gov Media 77755 Download
Data to support the BLA will be submitted by the companies to the FDA.
Bla submission fda. Action Date Submission Supplement Categories or Approval Type Letters Reviews Labels Patient Package Insert Note Url. Data to support the BLA will be submitted by the Companies to the FDA on a rolling basis over the coming weeks with a request for Priority Review. Data to support the BLA will be submitted by the Companies to the FDA on a rolling basis over the coming weeks with a request for Priority Review.
You can submit a BLA for a specified biotechnological product and an NDA or an ANDA for all drug products in the CTD format. The application can either be in paper or eCTD format. For administrative purposes designate this submission Final Printed Carton and Container Labels for approved BLA 761040 Approval of this submission by FDA is not required before the labeling is used.
FDA and Industry Actions on Premarket Notification 510k Submissions. BNTX today announced the initiation of a Biologics License Application BLA with the US. Guidance for Industry and Food and Drug Administration Staff CDRHCBER October 2017.
Abbreviated New Drug Application ANDA An Abbreviated New Drug Application ANDA contains data that when submitted to FDAs Center for Drug Evaluation and Research Office of Generic Drugs. Pharmaceutical Product Applications and Related Submissions Using the eCTD Specifications May 2015 Revision 3. A submission to an NDA BLA or efficacy supplement that purports to answer all of the deficiencies that need to be addressed by the applicant.
Whereas a new drug application NDA is used for drugs subject to the drug approval provisions of the FDC Act a biologics license application BLA. Effect on FDA Review Clock and Goals. BLA applicants must submit Form FDA 356h to the Centre for Biologics Evaluation and Research CBER.
The test submission must contain at least Module 1 FDA Form 356h for NDABLAANDA or 1571 for IND no form for DMF cover letter and all XML components Select CDER as the Center Select ECTD as Submission Type Use any 6-digit number as the test application number Select an eCTD. BLA Clinical Review Memorandum. A BLA is submitted by any legal person or entity who is engaged in manufacture or an applicant for a license who takes responsibility for compliance with.
The Prescription Drug User Fee Act PDUFA goal. PFE and BioNTech SE Nasdaq. Data to support the BLA will be submitted by the companies to the FDA on a rolling basis over the coming weeks with a request for Priority Review.
The Prescription Drug User Fee Act PDUFA goal. Application Type Original application STN 125703 CBER Received Date 11 December 2019 PDUFA Goal Date 10 August 2020 Division Office. Submission of a BLA which requires longer-term.
Since then the Companies have delivered more than 170 million doses of the vaccine across the US. Division of Clinical. Data to support the BLA will be submitted by the Companies to the FDA on a rolling basis over the coming weeks with a request for Priority Review.
NEW YORK MAINZ Germany--BUSINESS WIRE-- Pfizer Inc. FDA form 356h. The Prescription Drug User Fee Act PDUFA goal.
The Prescription Drug User Fee Act PDUFA goal.
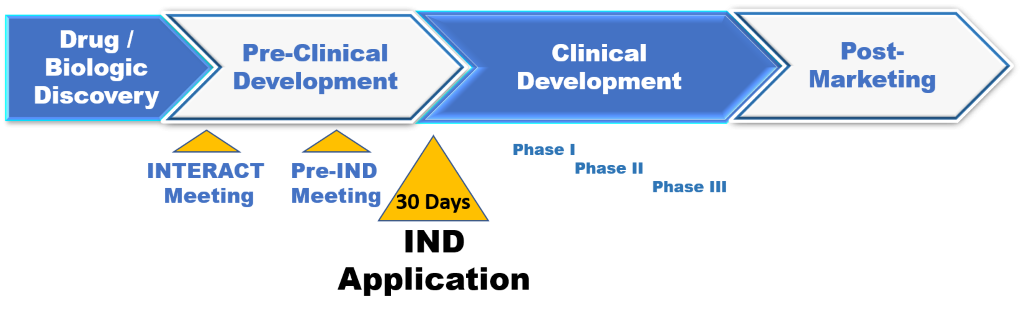 Common Problems To Avoid With Ind Applications For New Drugs And Biologics Criterion Edge
Common Problems To Avoid With Ind Applications For New Drugs And Biologics Criterion Edge
 From Drug Discovery To Approval Phase Iv Biotech Primer Weekly
From Drug Discovery To Approval Phase Iv Biotech Primer Weekly
 Prerequisites For Bla Approval Process Bla Submissions Fda 356h Biologics
Prerequisites For Bla Approval Process Bla Submissions Fda 356h Biologics
Https Www Fdli Org Wp Content Uploads 2020 10 Hegreness Matthew Pdf
 Fda May Refuse To File Your Nda Bla Submission Asphalion
Fda May Refuse To File Your Nda Bla Submission Asphalion
Https Www Fda Gov Media 78941 Download
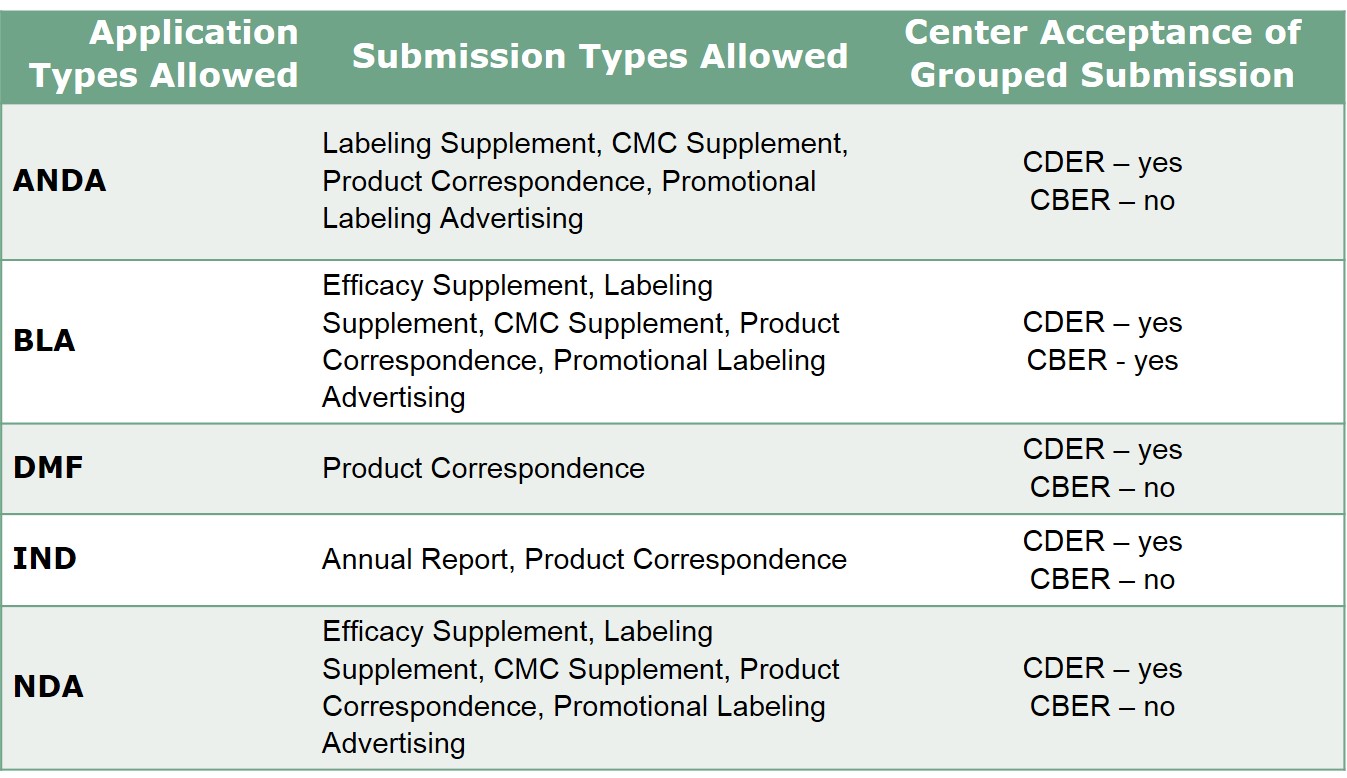 Fda Module 1 Grouped Submissions Q A Certara
Fda Module 1 Grouped Submissions Q A Certara
 Drug Development Process Abbreviations Bla Biologics License Download Scientific Diagram
Drug Development Process Abbreviations Bla Biologics License Download Scientific Diagram
 Drug Development Process Abbreviations Bla Biologics License Download Scientific Diagram
Drug Development Process Abbreviations Bla Biologics License Download Scientific Diagram
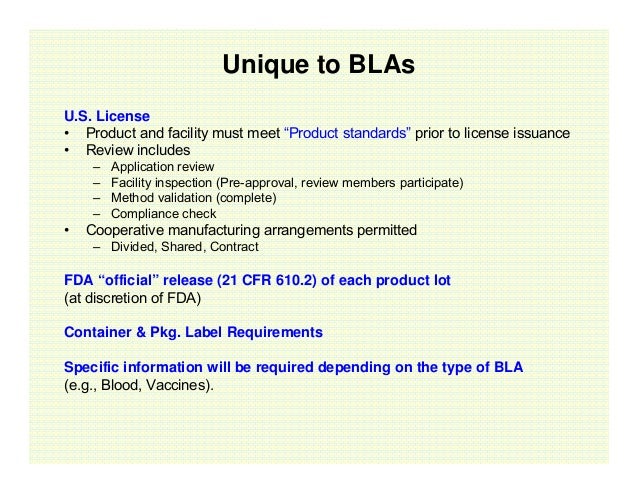
The Biologics License Application Bla Process Explained
 Drug Development Process Abbreviations Bla Biologics License Download Scientific Diagram
Drug Development Process Abbreviations Bla Biologics License Download Scientific Diagram



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.