This is an open-label phase12 multi-center trial to evaluate the efficacy safety tolerability and immunogenicity of INO-3107 in participants with HPV-6 andor HPV-11 associated recurrent respiratory papillomatosis RRP. Following the approval the company has commenced a Phase I clinical trial of the vaccine candidate in healthy participants.
News Ivi Ties Up With Snuh For Clinical Trial Of Covid 19 Vaccine Seoul National University Hospital
Inovio Covid-19 vaccine shows promise in early-stage trial 01 Jul 2020 Last Updated July 1st 2020 0716 Inovio Pharmaceuticals has reported positive interim results from the initial two cohorts in Phase I clinical trial of its Covid-19 vaccine candidate INO-4800.

Inovio clinical trials. Ulrike Leone from Pixabay. Interventional Clinical Trial Actual Enrollment. INOVIO is also assessing nonclinical efficacy of INO-4800 in several animal challenge models with Public Health England PHE and Commonwealth Scientific and Industrial Research Organization CSIRO in Australia.
The company has already produced thousands of INO-4800 doses for Phase I and II trials. For more than a decade INOVIO has been working closely with regulators to assess DNA medicines for their potential in disease prevention and treatment especially for emerging infectious diseases. INOVIO is also evaluating INO-4800 a DNA vaccine candidate against COVID-19 in a Phase 2 clinical trial in the US as well as Phase 2 trials in China and South Korea.
The International Vaccine Institute IVI and Seoul National University SNU Hospital have partnered to conduct a Phase III clinical trial of Inovio Pharmaceuticals Covid-19 vaccine INO-4800 in South Korea. INO-4800 is a DNA vaccine candidate that can potentially provide protection against the SARS-CoV-2 virus that causes Covid-19. The trial population is divided into two cohorts.
See listed clinical studies related to the coronavirus disease COVID-19 ClinicalTrialsgov is a resource provided by the US. National Library of Medicine. Partners and collaborators include Advaccine ApolloBio Corporation AstraZeneca The Bill Melinda Gates Foundation Coalition for Epidemic Preparedness Innovations CEPI Defense Advanced Research.
With more than 3000 patients receiving INOVIO investigational DNA medicines in more than 7000 applications across a range of clinical trials INOVIO has a strong track record of rapidly. Biotech firm Inovio is set to go ahead with the Phase II segment of its planned Phase IIIII trial of its Covid-19 vaccine candidate INO-4800 following clearance from the US Food and Drug Administration FDA. A Prospective Randomized Double-Blind Placebo-Controlled Phase 3 Study of VGX-3100 Delivered Intramuscularly Followed by.
INO- 4800 contains the plasmid pGX9501 which encodes for the full length of the Spike glycoprotein of SARS-CoV-2. INOVIO has partnered with Advaccine and the International Vaccine Institute to conduct clinical trials of INO-4800 in China and South Korea respectively. INOVIO has demonstrated that DNA medicines can be delivered directly into cells in the body via a proprietary smart device to safely produce a robust immune response in clinical trials involving more than 2000 patients in 7000 administrations.
A press release from the company last week stated that The Phase 3 segment of the INNOVATE remains on partial clinical hold until INOVIO satisfactorily resolves the. This is a phase IIIa trial to evaluate the safety tolerability and immunological profile of INO-4800 administered by intradermal ID injection followed by electroporation EP using the CELLECTRA 2000 device in healthy adults aged 19 to 64 years in Republic of Korea. INOVIO is also evaluating INO-4800 a DNA vaccine candidate against COVID-19 in a Phase 2 clinical trial in the US as well as Phase 2 trials in China and South Korea.
Explore 377550 research studies in all 50 states and in 220 countries. Inovio Covid-19 vaccine candidate was generally safe and well-tolerated. With more than 3000 patients receiving INOVIO investigational DNA medicines in more than 7000 applications across a range of clinical trials INOVIO has a strong track record of rapidly generating DNA medicine candidates with potential to meet urgent global health needs.
Quadruple Participant Care Provider Investigator Outcomes Assessor Primary Purpose. We are grateful for their guidance and rapid response as we develop our COVID-19 DNA vaccine in particular as we advance from funding to initiation of our first-in-human trial in ten weeks a major milestone for INOVIO. The advantages of INOVIOs DNA medicines platform in a pandemic situation like the world is now facing with COVID-19 are how fast DNA medicines can be.
Participants with diagnoses of Juvenile-Onset RRP as defined by age at first diagnosis of RRP 12 years. This is an open-label trial to evaluate the safety tolerability and immunological profile of INO-4800 administered by intradermal ID injection followed by electroporation EP using CELLECTRA 2000 device in healthy adult volunteers. Inovio Pharmaceuticals has received approval from the US Food and Drug Administration FDA for the investigational new drug IND application of its DNA vaccine candidate INO-4800 against Covid-19.
 Ivi Partners With Seoul National University Hospital To Start Phase 1 2 Clinical Trial Of Inovio S Covid 19 Dna Ino 4800 Vaccine In South Korea Ivi
Ivi Partners With Seoul National University Hospital To Start Phase 1 2 Clinical Trial Of Inovio S Covid 19 Dna Ino 4800 Vaccine In South Korea Ivi
 Inovio Pharmaceuticals And Beijing Advaccine Biotechnology Ino 4800
Inovio Pharmaceuticals And Beijing Advaccine Biotechnology Ino 4800
 For Patients Clinical Trials Inovio Pharmaceuticals
For Patients Clinical Trials Inovio Pharmaceuticals
Is Inovio Pharmaceuticals Inc Late Reporting Eu Clinical Trials
 Inovio Pharmaceuticals Boosts Covid 19 Vaccine Manufacturing
Inovio Pharmaceuticals Boosts Covid 19 Vaccine Manufacturing
 Covid 19 Inovio Pharmaceuticals
Covid 19 Inovio Pharmaceuticals
 Ivi Inovio And Knih Partner With Cepi In A Phase I Ii Trial Of Inovio S Covid 19 Vaccine Eurekalert Science News
Ivi Inovio And Knih Partner With Cepi In A Phase I Ii Trial Of Inovio S Covid 19 Vaccine Eurekalert Science News
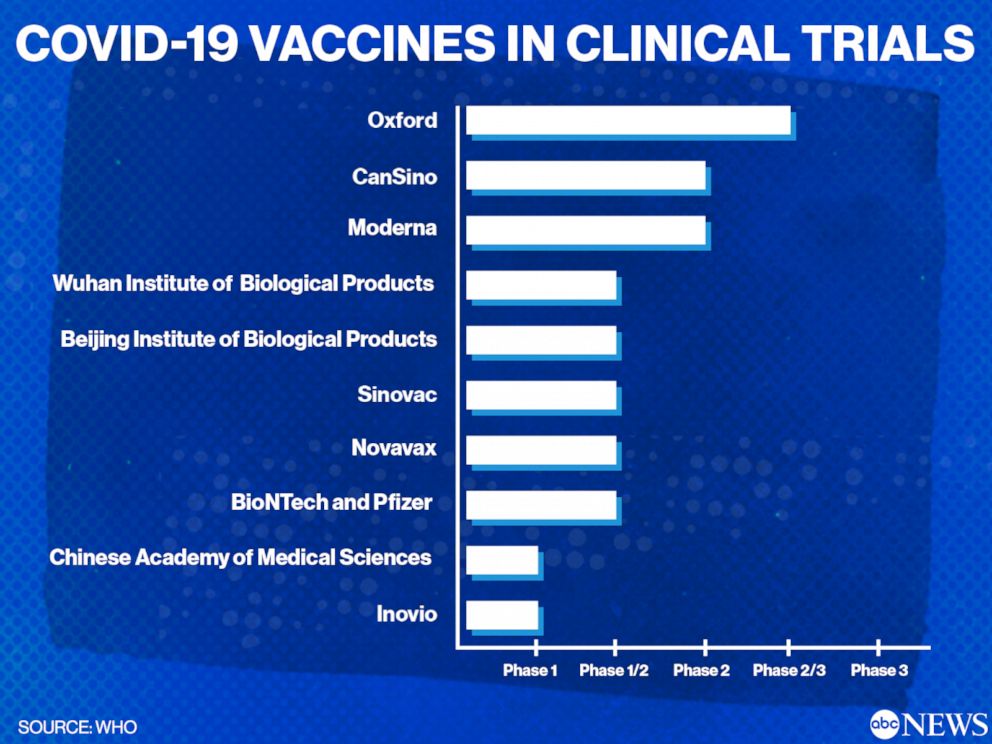 Out Of The Lab And Into People S Arms A List Of Covid 19 Vaccines That Are Being Studied In Clinical Trials Abc News
Out Of The Lab And Into People S Arms A List Of Covid 19 Vaccines That Are Being Studied In Clinical Trials Abc News
 Inovio Reports Positive Data From Phase Ii Covid 19 Dna Vaccine Trial
Inovio Reports Positive Data From Phase Ii Covid 19 Dna Vaccine Trial

Inovio Pharmaceuticals Prostate Cancer Dna Vaccine Demonstrates Strong T Cell Responses In Monkeys Company On Track For Phase I Clinical Trial
 Covid 19 Vaccines Are Moving Toward Clinical Trials
Covid 19 Vaccines Are Moving Toward Clinical Trials
 Inovio Expands Manufacturing Of Covid 19 Dna Vaccine Ino 4800 With New Funding From Cepi Richter Helm Biologics
Inovio Expands Manufacturing Of Covid 19 Dna Vaccine Ino 4800 With New Funding From Cepi Richter Helm Biologics


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.