Transfusion-dependent β-thalassemia TDT and sickle cell disease SCD are severe monogenic diseases with severe and potentially life-threatening manifestations. We performed electroporation of CD34 hematopoietic stem and progenitor cells obtained from healthy donors with CRISPR.
 Crispr Cas9 A Preclinical And Clinical Perspective For The Treatment Of Human Diseases Molecular Therapy
Crispr Cas9 A Preclinical And Clinical Perspective For The Treatment Of Human Diseases Molecular Therapy
Since receiving a landmark treatment with the gene-editing tool CRISPR a sickle cell patient has the strength to care for herself and her children while navigating the pandemic.

Crispr gene therapy sickle cell. The cells were differentiated from bone marrow with unedited and edited hematopoietic stem cells and the red arrows show the sickled cells. CRISPR gene therapy shows promise against blood diseases. The first two patients to receive a CRISPR-based treatment for the inherited blood disorders sickle cell disease and beta thalassemia have benefited from the experimental therapy and experienced.
Next scientists used CRISPR. A CRISPR Approach to Treating Sickle Cell. In the paper published in the New England Journal of Medicine CRISPR-Cas9 Gene Editing for Sickle Cell Disease and beta-Thalassemia researchers reported gene editing modified the DNA of stem.
Sickle cell disease is caused by a single change in the DNA code of the beta-globin gene. Studies published in 2016 described a successful proof-of-concept in treating sickle cell disease in mice using the CRISPR-Cas9 gene editing tool. Another sickle cell disease clinical trial that uses the CRISPR gene-editing tool to turn on a fetal form of hemoglobin reported promising results.
Recent discovery of CRISPR. Researchers report early successes using genetic approaches to treat sickle-cell anaemia and β-thalassaemia. Fyodor Urnov IGIs scientific director of technology and translation has led the basic research on CRISPR therapies for sickle cell disease.
Appeared in BioNews 1090. Red blood cells from patient with sickle cell disease. Treating Sickle Cell Disease with Genetic Editing Tools.
To lead the development manufacturing and commercialization of gene editing therapy CTX-001 for sickle cell disease SCD and. The new trial uses the CRISPR-Cas9 nuclease a fully assembled Cas9 protein and guide RNA sequence targeting. Last Updated April 1 2021 CRISPR genome editing technology which was developed at UC Berkeley has been approved for clinical trials to correct gene mutations responsible for sickle cell disease.
BCL11A is a transcription factor that represses γ-globin expression and fetal hemoglobin in erythroid cells. We performed electroporation of CD34 hematopoietic stem and progenitor cells obtained. CRISPR gene therapy for sickle cell disease approved by the FDA.
After six years of work that experimental treatment has now been approved for clinical trials by the US. Rapid and substantial progress in genome editing approaches have proven valuable as a curative option given plausibility to either correct the underlying mutation in patient-derived hematopoietic stemprogenitor cells HSPCs induce fetal hemoglobin expression to circumvent sickling of red blood cells RBCs or create corrected induced pluripotent stem cells iPSCs among other approaches. By Dr Molly Godfrey.
Whatever the successful strategy either ex vivo or in vivo the CRISPR platform developed for sickle cell disease could transform gene therapy for other diseases. To try to treat Grays sickle cell doctors started by removing bone marrow cells from her blood last spring. In biotech and biopharmas third-largest ever up-front development and commercialization deal Crispr Therapeutics AG will receive an initial 900 million in an amended deal with Vertex Pharmaceuticals Inc.
As sickle cell disease is a well known genetic disorder it is considered a leading candidate for gene editing therapies. Food and Drug Administration enabling the first tests in humans of a CRISPR-based therapy to directly correct the mutation in the beta-globin gene responsible for sickle cell disease. A clinical trial for a new gene therapy approach to treat sickle cell disease has been approved to proceed by the US Food and Drug Administration.
BCL11A is a transcription factor that represses γ-globin expression and fetal hemoglobin in erythroid cells. The trial will combine CRISPR technology developed at Innovative Genomics Institute IGI a joint UC Berkeley-UCSF initiative founded by Berkeleys Nobel Prize-winning scientist Jennifer Doudna PhD with UCLAs expertise in genetic analysis and cell manufacturing and the decades-long expertise at UCSF Benioff Childrens Hospital Oakland in. Posted on April 2nd 2019 by Dr.
Beta-globin is one of the proteins in the hemoglobin complex.
 Crispr Cas9 Gene Editing For Sickle Cell Disease And B Thalassemia Nejm
Crispr Cas9 Gene Editing For Sickle Cell Disease And B Thalassemia Nejm
 Nih Researchers Create New Viral Vector For Improved Gene Therapy In Sickle Cell Disease National Institutes Of Health Nih
Nih Researchers Create New Viral Vector For Improved Gene Therapy In Sickle Cell Disease National Institutes Of Health Nih
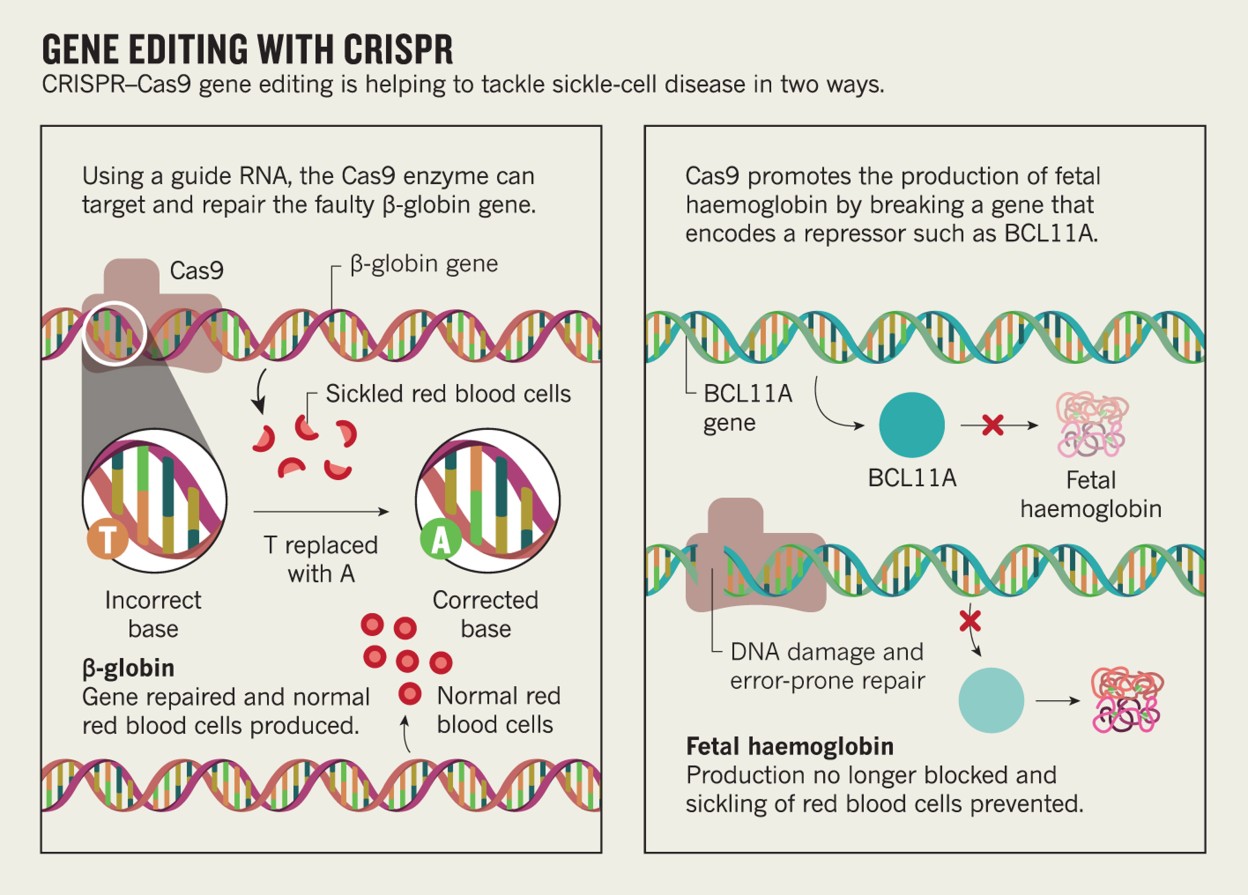 Gene Therapy Erasing Sickle Cell Disease Nature
Gene Therapy Erasing Sickle Cell Disease Nature
 New Genetic Weapons Challenge Sickle Cell Disease Eurekalert Science News
New Genetic Weapons Challenge Sickle Cell Disease Eurekalert Science News
 Crispr Cas9 Gene Editing For Sickle Cell Disease And B Thalassemia Nejm
Crispr Cas9 Gene Editing For Sickle Cell Disease And B Thalassemia Nejm
 Crispr Is Coming To The Clinic This Year
Crispr Is Coming To The Clinic This Year
 Crispr Cas9 Genome Engineering In Hematopoietic Cells Sciencedirect
Crispr Cas9 Genome Engineering In Hematopoietic Cells Sciencedirect
 The First Crispr Gene Therapy To Cure Sickle Cell Disease Advanced Science News
The First Crispr Gene Therapy To Cure Sickle Cell Disease Advanced Science News
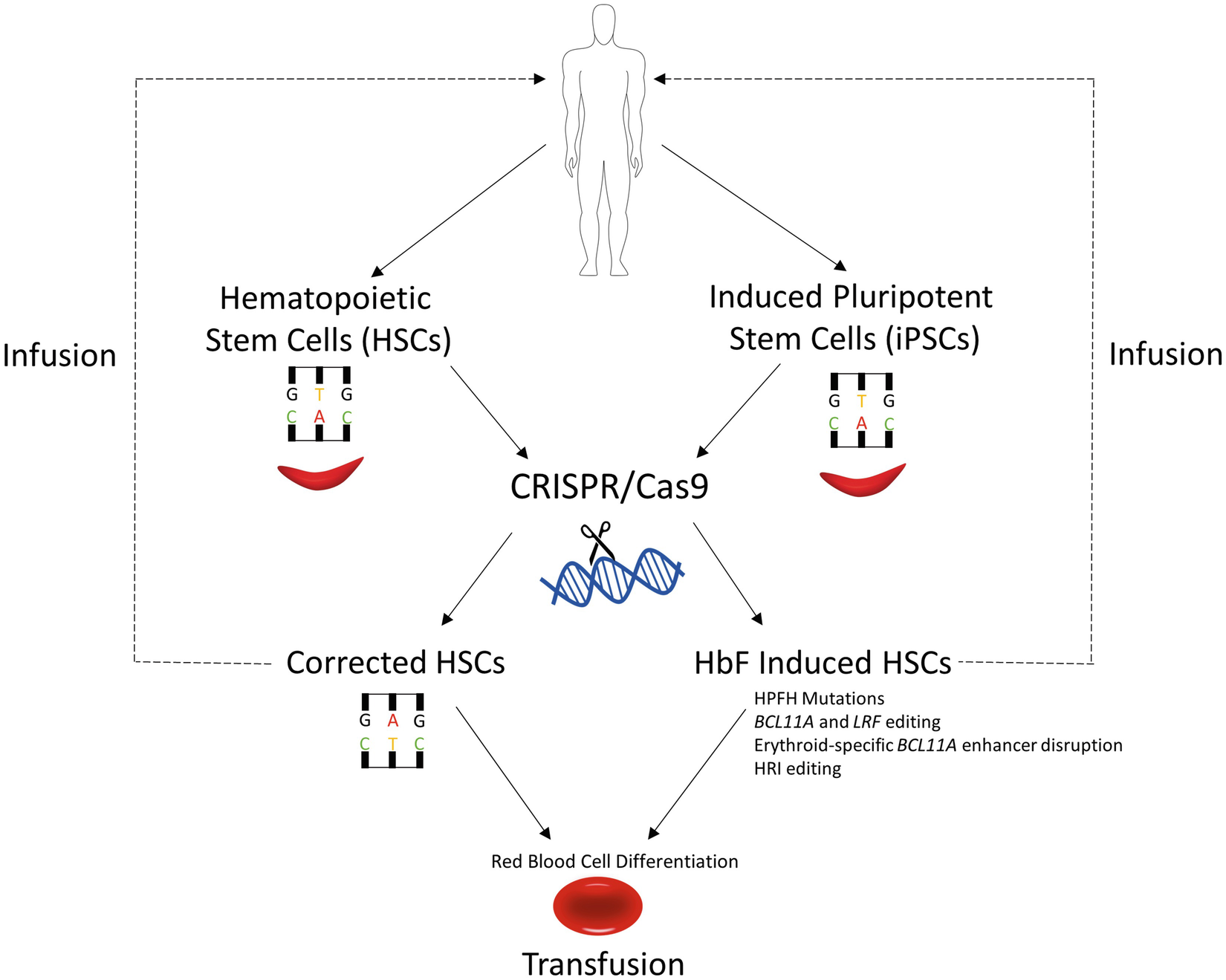 Crispr Cas9 For Sickle Cell Disease Applications Future Possibilities And Challenges Springerlink
Crispr Cas9 For Sickle Cell Disease Applications Future Possibilities And Challenges Springerlink
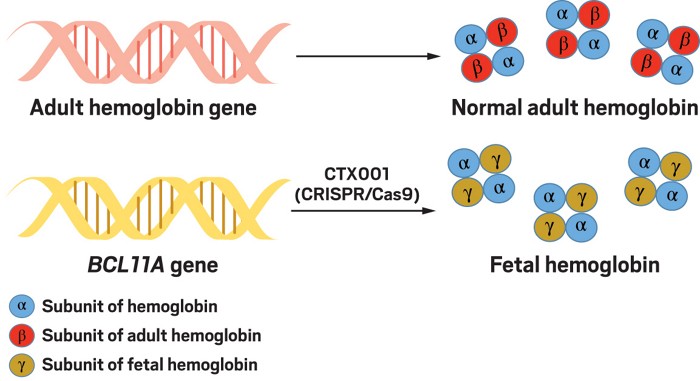 Crispr Gene Editing In Humans Appears Safe And Potentially Effective
Crispr Gene Editing In Humans Appears Safe And Potentially Effective
 Crispr Therapeutics Plans First Crispr Clinical Trial In Europe For 2018
Crispr Therapeutics Plans First Crispr Clinical Trial In Europe For 2018
 Editing The Sickle Cell Disease Mutation In Human Hematopoietic Stem Cells Comparison Of Endonucleases And Homologous Donor Templates Molecular Therapy
Editing The Sickle Cell Disease Mutation In Human Hematopoietic Stem Cells Comparison Of Endonucleases And Homologous Donor Templates Molecular Therapy
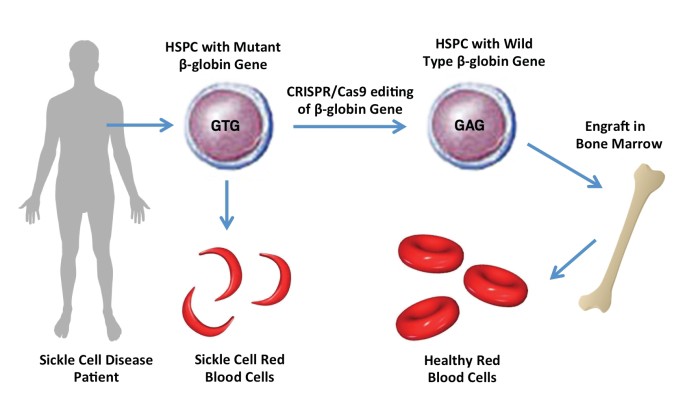 Crispr Edits Sickle Cell Mutation
Crispr Edits Sickle Cell Mutation
Crispr Gene Editing In Humans Appears Safe And Potentially Effective

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.